GMP and non-GMP plasmid production for pre-clinical, clinical and commercial applications

GMP Plasmid Production
NorthX Biologics is dedicated to providing GMP grade plasmid DNA for clinical and commercial applications. Our plasmid manufacturing meets full Good Manufacturing Practice (GMP) standards in qualified clean rooms, ensuring the highest level of quality and safety for use in applications such as DNA vaccines, direct-delivered gene therapy, and late-phase viral vector products.
- Fully single-use process equipment
- Fully traceable non-animal origin raw materials
- RNase-free process
- Drug substance quality
- GMP level analytics and certificate of analysis (CoA)
- EU release via on-site qualified person (QP)
- Clinical and commercial scale
High Quality Plasmid Production
NorthX Biologics offers High Quality grade plasmid DNA for pre-clinical applications in vitro or in vivo, including toxicology studies. Our High Quality plasmid DNA is manufactured in accordance with regulatory expectations for critical starting material for cell and gene therapies, making it suitable for the production of viral vectors for clinical use. By utilizing our defined platform technology, we provide a fast turnaround from the start to the delivery of the plasmid.
- Critical starting material for cell and gene therapies
- mRNA Templates
- Aligned high quality level analytics
- Segregated laboratories
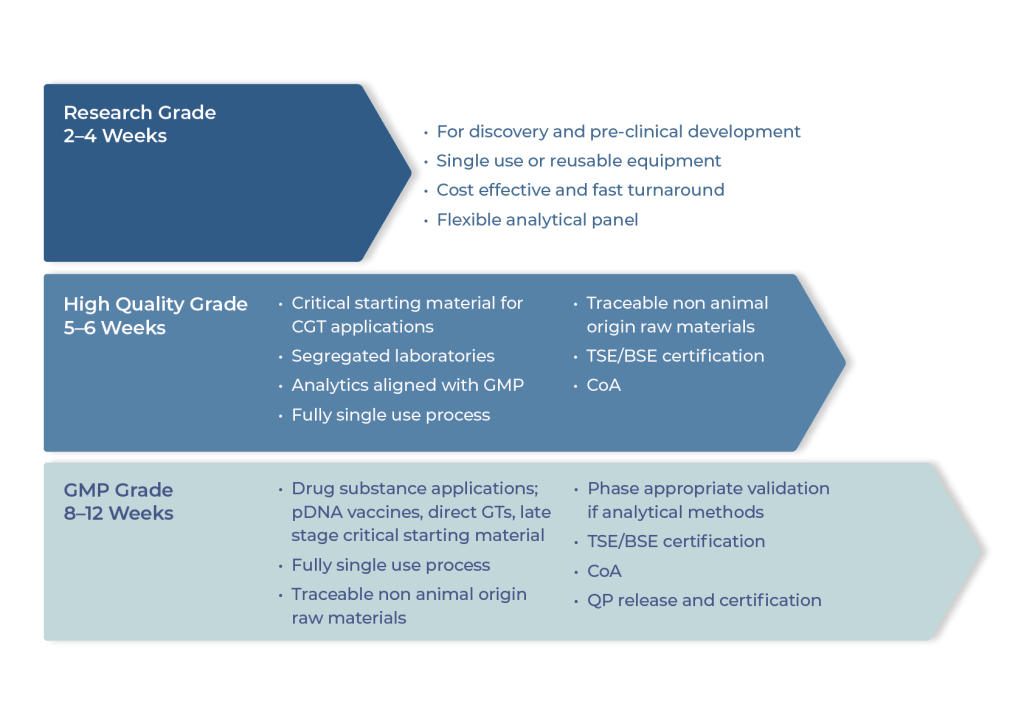
Plasmid DNA solutions for all phases
We offer a range of plasmid qualities with the right level of control for every purpose, ensuring efficient expedition of your program with full regulatory compliance.
We love plasmid DNA!
Have a need for plasmid DNA supply? Our quality levels can match any application for plasmid DNA and we can support you for all phases of your product life cycle. Let us know how we can help and let’s journey together.